The valence electrons occupy higher levels due to the increasing quantum number (n). As a result, the valence electrons are further away from the nucleus as ‘n’ increases. Electron shielding prevents these outer electrons from being attracted to the nucleus; thus, they are loosely held, and the resulting atomic radius is large. Some books and dictionaries define valence electrons as 'outer shell electrons that participate in chemical bonding' and by this definition, elements can have more than 8 valence electrons as explained by F'x. Some books and dictionaries define valence electrons as 'electrons in the highest principal energy level'.
Also found in: Thesaurus, Medical, Acronyms, Encyclopedia, Wikipedia.Va·lence
(və-läNs′, vă-) A city of southeast France on the Rhone River south of Lyon. Settled in Roman times, it was captured by the Visigoths in ad 413 and the Arabs c. 730.
va·lence
(vā′ləns) also va·len·cy(-lən-sē)n.pl.val·lenc·es also val·len·cies1. Chemistrya. The combining capacity of an atom or group of atoms as determined by the number of electrons it can lose, add, or share when it reacts with other atoms or groups. Also called oxidation state.
b. An integer used to represent this capacity, which may be given as positive or negative depending on whether electrons are lost or gained, respectively: The valences of copper are +1 and +2.
2. The number of binding sites of a molecule, such as an antibody or antigen.
3. The number of different antigens contained in a vaccine, corresponding to the number of pathogens that it is active against.
4. Psychology The degree of attraction or aversion that an individual feels toward a specific object or event.
5. Linguistics The number and type of arguments that a lexical item, especially a verb, can combine with to make a syntactically well-formed sentence, often along with a description of the categories of those constituents. Intransitive verbs (appear, arrive) have a valence of one—the subject; some transitive verbs (paint, touch), two—the subject and direct object; other transitive verbs (ask, give), three—the subject, direct object, and indirect object.
6. The capacity of something to unite, react, or interact with something else: 'I do not claim to know much more about novels than the writing of them, but I cannot imagine one set in the breathing world which lacks any moral valence'(Robert Stone).
[Latin valentia, capacity, from valēns, valent-, present participle of valēre, to be strong; see wal- in Indo-European roots.]
American Heritage® Dictionary of the English Language, Fifth Edition. Copyright © 2016 by Houghton Mifflin Harcourt Publishing Company. Published by Houghton Mifflin Harcourt Publishing Company. All rights reserved.
valence
(ˈveɪləns) n1. (Chemistry) another name (esp US and Canadian) for valency
2. (Chemistry) the phenomenon of forming chemical bonds
Valence
(French valɑ̃s)
 n
n (Placename) a town in SE France, on the River Rhône. Pop: 64 260 (1999)
Collins English Dictionary – Complete and Unabridged, 12th Edition 2014 © HarperCollins Publishers 1991, 1994, 1998, 2000, 2003, 2006, 2007, 2009, 2011, 2014
va•lence
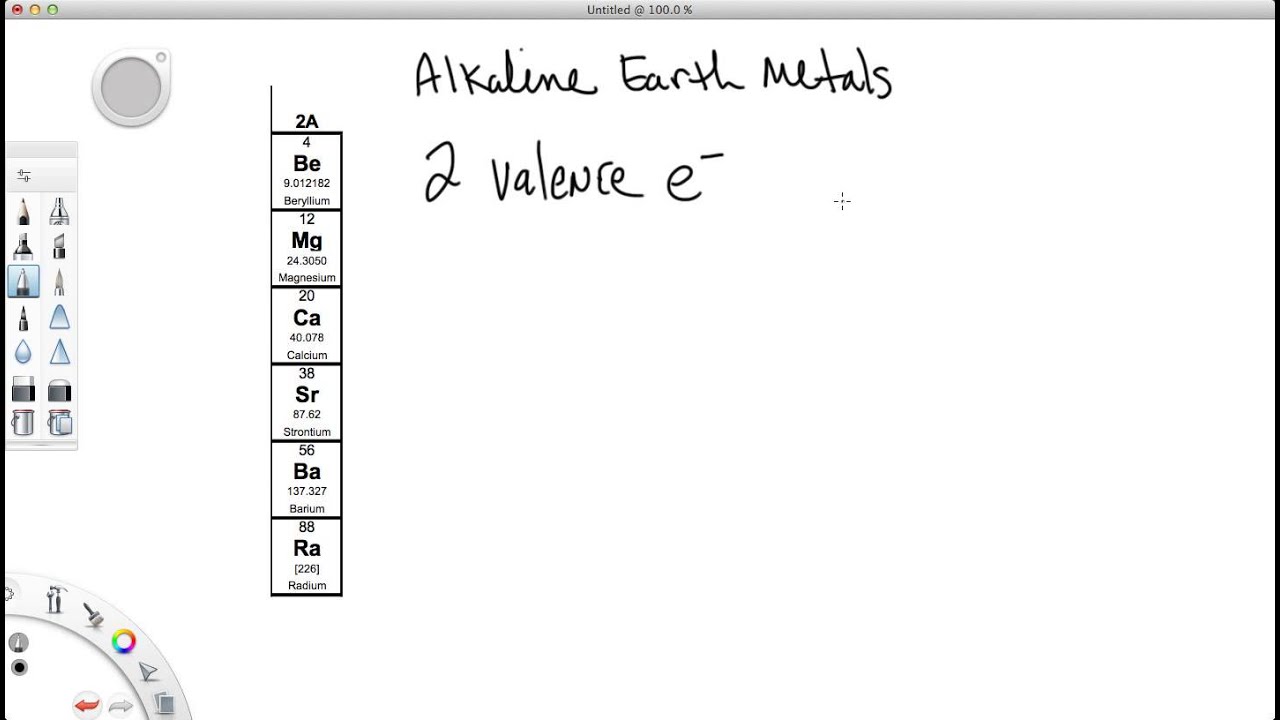
How Many Valence Electrons Are In Se
(ˈveɪ ləns)also valency
n. 1.
a. the quality that determines the number of atoms or groups with which any single atom or group will unite chemically.
b. the relative combining capacity of an atom or group compared with that of the standard hydrogen atom.
2. the number of binding sites on a molecule, as an antibody or antigen.
[1865–70; < Latin valentia strength, worth =valent-, s. of valēns, present participle of valēre to be strong + -ia n. suffix; see -ence]
Va•lence
(væˈlɑ̃s)n.
Random House Kernerman Webster's College Dictionary, © 2010 K Dictionaries Ltd. Copyright 2005, 1997, 1991 by Random House, Inc. All rights reserved.
va·lence
(vā′ləns) A whole number that represents the ability of an atom or a group of atoms to combine with other atoms or groups of atoms. The valence is determined by the number of electrons that an atom can lose, add, or share. A carbon atom, for example, can share four electrons with other atoms and therefore has a valence of 4.
The American Heritage® Student Science Dictionary, Second Edition. Copyright © 2014 by Houghton Mifflin Harcourt Publishing Company. Published by Houghton Mifflin Harcourt Publishing Company. All rights reserved.
| Noun | 1. | valence - (biology) a relative capacity to unite or react or interact as with antigens or a biological substrate power, powerfulness - possession of controlling influence; 'the deterrent power of nuclear weapons'; 'the power of his love saved her'; 'his powerfulness was concealed by a gentle facade' biological science, biology - the science that studies living organisms |
| 2. | valence - (chemistry) a property of atoms or radicals; their combining power given in terms of the number of hydrogen atoms (or the equivalent) covalence, covalency - valence characterized by the sharing of electrons in a chemical compound; the number of pairs of electrons an atom can share power, powerfulness - possession of controlling influence; 'the deterrent power of nuclear weapons'; 'the power of his love saved her'; 'his powerfulness was concealed by a gentle facade' chemical science, chemistry - the science of matter; the branch of the natural sciences dealing with the composition of substances and their properties and reactions |
Se Number Of Valence Electrons
Based on WordNet 3.0, Farlex clipart collection. © 2003-2012 Princeton University, Farlex Inc.
Collins Spanish Dictionary - Complete and Unabridged 8th Edition 2005 © William Collins Sons & Co. Ltd. 1971, 1988 © HarperCollins Publishers 1992, 1993, 1996, 1997, 2000, 2003, 2005
valence
, valencyn (Chem) → Wertigkeitf, → Valenzf; (Ling) → Valenzf

Collins German Dictionary – Complete and Unabridged 7th Edition 2005. © William Collins Sons & Co. Ltd. 1980 © HarperCollins Publishers 1991, 1997, 1999, 2004, 2005, 2007
Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.
Link to this page:
