- Name: Bromine Symbol: Br Atomic Number: 35 Atomic Mass: 79.904 amu Melting Point:-7.2 °C (265.95 K, 19.04 °F) Boiling Point: 58.78 °C (331.93 K, 137.804 °F) Number of Protons/Electrons: 35 Number of Neutrons: 45 Classification: Halogen Crystal Structure: Orthorhombic Density @ 293 K: 3.119 g/cm 3 Color: Red Atomic Structure.
- A monatomic bromine has 7 valence electrons so it interacts with itself and shares electrons (covalent binding) to end up with 8 electrons around each atom. This is the reason why bromine floats around as a diatom.
Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. The chemical symbol for Bromine is Br. Bromine is the third-lightest halogen, and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas.
How many electrons are there in #Br^-#?
1 Answer
Number Of Electrons Br
Explanation:
The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A neutral bromine atom would also have 35 electrons. In order for a bromine atom to become a
Br Electrons Number
Below is the Lewis dot structure for a neutral bromine atom, which has seven valence electrons.
Below is the Lewis dot structure for a
Br Electrons Valence
The diagram below shows how a bromine atom gains an electron from the element lithium in order to form the ionic compound LiBr.
Related questions
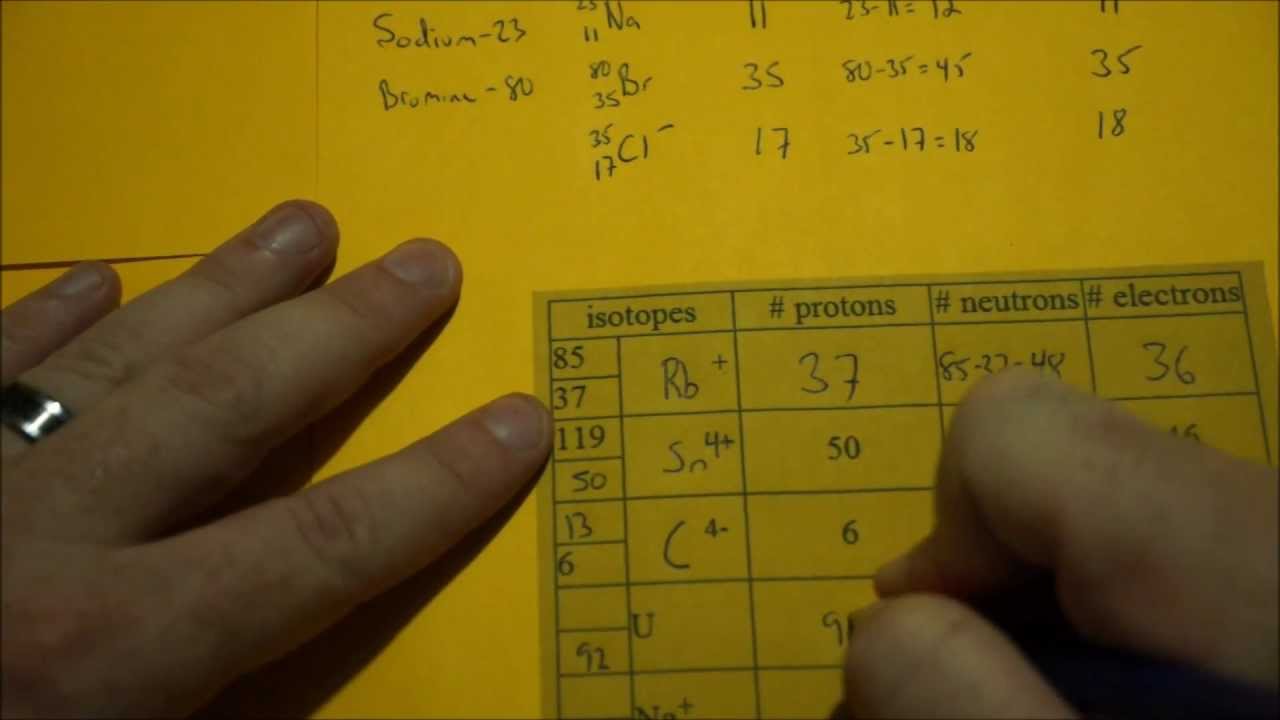
How do you find the protons, neutrons, and electrons for a Bromine (#1^-#) anion with a mass number of 80?
1 Answer
79 Br Electrons
The atomic number is the number of Protons add one for the number of electrons and 80 - 35 is the number of neutrons.
Explanation:
The atomic number is the number of protons. This is the number that never changes for an element (or else the chemical properties will change!).
For Bromine the atomic number is 35. so number of protons = 35
In a neutral atom the number of protons = the number of electrons. 35 protons = 35 electrons. But Bromine anion with a charge of -1 has one extra electron so 35 +1 = 36 electrons.
36 electrons is the number of electrons in the stable inert gas Krypton. (That's why ions are more stable than atoms and atoms tend to form ions.)
The atomic mass is the sum of the number of protons and the number of neutrons (as electrons weigh almost nothing) so
Protons + neutrons = Mass putting in known values
Bromine Valence Electrons
Br Electrons Lost Or Gained
Be Valence Electrons
n = 45
So Bromine
Related questions
