- Scandium is a soft, silvery metallic element. Its atomic number is 21, making it the lightest of the transition metals. Scandium is not particularly rare - its occurrence in crustal rocks is 22 around ppm. This makes scandium generally more abundant than lead, mercury, and precious metals.
- Scandium (Sc) has an atomic number of twenty-one. This member of the transition elements group is a silver-white that has been historically categorized as a rare earth element. Interesting Scandium Facts.
These are the d-block elements or first row transition elements. It starts with Scandium (Sc) with atomic number 21 followed by Titanium (Ti) atomic number 22 and then Vanadium (V) with atomic number 23 and so on.
Chemical properties of scandium - Health effects of scandium - Environmental effects of scandium
| |||||||||||||||||||||||||||||
Scandium Scandium is a soft, silvery transition element which occurs in rare minerals from Scandinavia. It develops a slightly yellowish or pinkish cast when exposed to air. Scandium tarnished in air and burn easily, once it has been ignited. It reacts with water to form hydrogen gas and will dissolve in many acids. Pure scandium is produced by heating scandium fluoride (ScF3) with calcium metal. | |||||||||||||||||||||||||||||
Scandium has no biological role. Only trace amounts reach the food chain, so the average person's daily intake is less than 0.1 microgram. Scadium is not toxic, although there have been suggestions that some of its compounds might be cancerogenic. Scandium is mostly dangerous in the working environment, due to the fact that damps and gasses can be inhaled with air. This can cause lung embolisms, especially during long-term exposure. Scandium can be a threat to the liver when it accumulates in the human body. |
Effects of scandium on the environment
Scandium is dumped in the environment in many different places, mainly by petrol-producing industries. It can also enter the environment when household equipment is thrown away. Scandium will gradually accumulate in soils and water soils and this will eventually lead to increasing concentrations in humans, animals and soil particles. |
Back to the periodic table of elements
More from 'Elements'
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com

Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Copyright © 1998-2021 Lenntech B.V. All rights reserved
Scandium was discovered by Lars Fredik Nilson in 1879. It is a transition element and is widely used as aluminum-scandium alloy for minor aerospace industry.
History and Discovery
The existence of scandium was predicted by Dmitri Mendeleev when he organized the elements in the periodic table. He called it ekaboron (boron like) and suggested some physical and chemical properties in 1869. Lars Fredrik Nilson and his team, in 1879 found this element in the minerals of euxenite and gadolinite. He prepared scandium oxide of high purity. The word scandium has been derived from Latin Scandia meaning Scandinavia [1]. In 1937, metallic scandium was prepared for the first time by Fischer and his colleagues through electrolysis of molten scandium, lithium and potassium chloride.
Scandium
| Periodic Table Classification | Group 3 Period 4 |
|---|---|
| State at 20C | Solid |
| Color | Silvery white |
| Electron Configuration | [Ar] 3d1 4s2 |
| Electron Number | 21 |
| Proton Number | 21 |
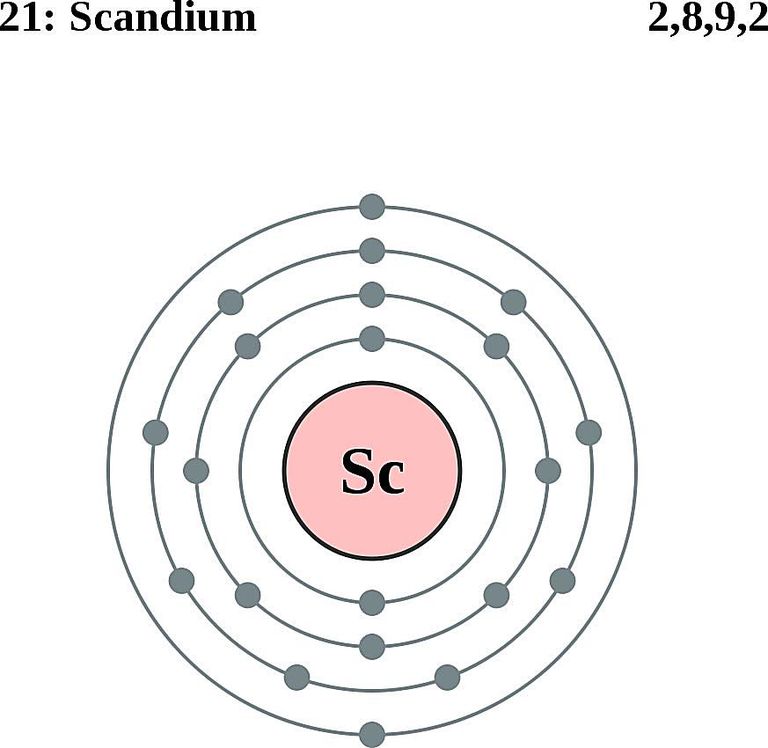
| Electron Shell | 2, 8, 9, 2 |
| Density | 2.99 g.cm-3 at 20°C |
| Atomic number | 21 |
| Atomic Mass | 44.95 g.mol -1 |
| Electronegativity according to Pauling | 1.36 |
Occurrence
Scandium is the 50th most common element in the earth crust and 35th most abundant element in the Sun. Scandium is found in ores of tin, uranium and tungsten. Thortveitite (Scandium silicate) contain 45% scandium in the form of scandium oxide but it is very rare in nature. Scandium is mostly produced as a by-product during the extraction of uranium through mineral davidite which contain around 0.02% scandium oxide. It is mainly mined in China, Kazakhstan, Madagascar, Norway and Russia.
Physical Characteristics
Scandium is a transition metal. It is silver-white soft metal. When exposed to air it develops a slightly yellowish or pinkish tint. It has a high melting point, but it is as light as aluminum. That means it has relatively low density, about 2.98g/cm3. Its melting point is 1541oC and boiling point is 2836oC. Scandium chemical symbol is Sc. Its atomic number is 21 and atomic weight is 44.95g/mol.
Chemical Characteristics
Scandium easily reacts with dilute acids. It is easily burnt and get tarnished in the presence of air. It reacts with water and form hydrogen gas. Pure form of scandium is produced by heating scandium fluoride with calcium metal. Scandium properties are similar with yttrium so it is often classified as a lanthanide like element. Its compound mostly exists in +3 oxidation state. Its oxides and hydroxide are amphoteric (ion that can react with both acid and base). Scandium halides are soluble in water except scandium fluoride which form impermeable passive layer. Organoscandium compounds have lower oxidation states like: 0, +1, +2.
Significance and Uses
- It is used in houses in equipment like color television, fluorescent lamps, energy saving lamps and glasses.
- It is also used to polish glass.
- Aluminum-Scandium alloys are used for aerospace industry.
- Scandium is used in sports equipment like bikes, baseball and bats etc.
- It is also helpful in reducing solidification cracking during welding of high strength aluminum alloys.
- Scandium oxide are used to make high intensity stadium light.
- Scandium iodide is used in mercury vapor lamps.
- Scandium-45 isotope is used in oil refineries.
- Scandium sulfate in diluted form used to improve the germination of seeds like corn, peas and wheat.
Health effects
Scandium is non-toxic in nature. And is has no known biological role. Certain compounds of scandium, however are highly hazardous for individuals working in laboratory areas and industries. Long term exposure of scandium may cause lungs embolism and liver damage.
Isotopes of Scandium
Naturally scandium is found in 45Sc isotope and it is stable. Thirteen radioactive isotopes have been identified, out of which the most stable one is 46Sc has half-life of 83.8 days, 47Sc has 3.35 days, 44Sc has 4 hours and 48Sc has 43.7 hours [3].
REFERENCES
[1]. Nilson, Lars Fredrik (1879). “Sur l’ytterbine, terre nouvelle de M. Marignac”. Comptes Rendus(in French). 88: 642–647.
[2]. Lide, David R. (2004). CRC Handbook of Chemistry and Physics. Boca Raton: CRC Press. pp. 4–28
[3]. Audi, Georges; Bersillon, O.; Blachot, J.; Wapstra, A. H. (2003). “The NUBASE Evaluation of Nuclear and Decay Properties”. Nuclear Physics A. Atomic Mass Data Center. 729: 3–128.
Proton Number Of Scandium
Other Periodic Table Elements
What Is Atomic Number Of Scandium
- Tennessine
Tennessine is a synthetic element that was discovered in 2010. It is highly radioactive and…
- Copernicium
Copernicium is an artificially produced element and was synthesized in 1996. It has many unstable…
- Holmium
Holmium was discovered in 1878. It has highest magnetic moment that is why it is…
